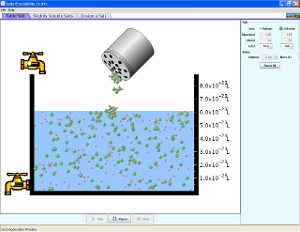
Directions: For this simulation you are going to be looking at the solubility of salt. Solubility refers to the ability of a substance to go into solution. Highly soluble substances go into solution very easily, while non-soluble substances do not. In other words, soluble substances dissolve easily.
Use the simulation embedded in this page to practice some of what you have learned about solubility. Play around with the amount of salt and water added, and observe what happens to the salt particles as they dissolve. Once you have tested multiple settings for the table salt simulation, test the less soluble salts using the same settings. Once you have done several simulations, answer the questions below.
Simulation
Questions
- Describe the behavior of the sodium and chlorine ions when the salt is first added to the solution.
- Describe what happens when the solution becomes saturated.
- What are the difference between the behavior of molecules of table salt and the behavior of molecules of a less soluble salt?